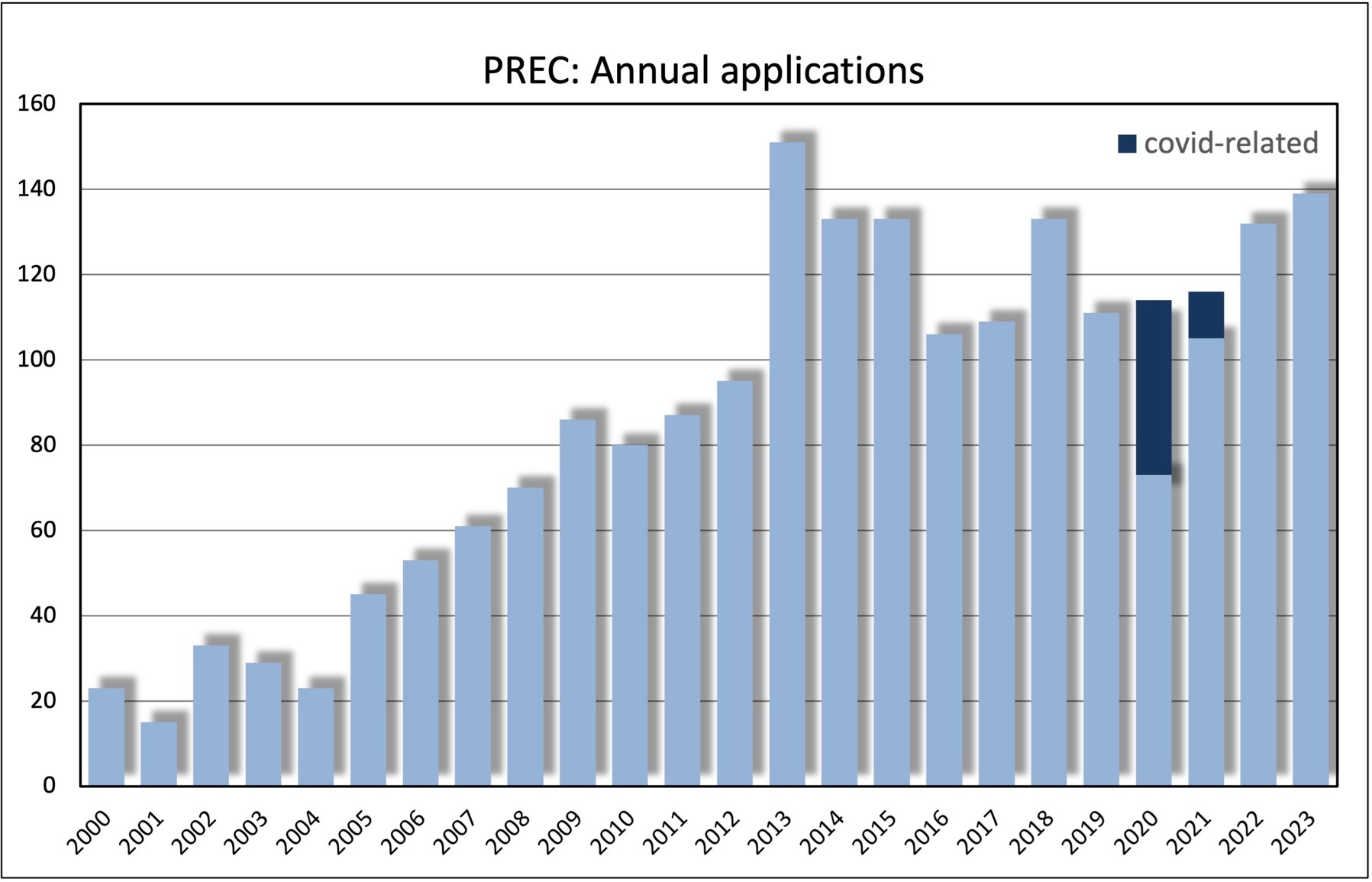
The Cambridge Psychology Research Ethics Committee considers applications for ethical review for research programmes in human psychology. Ethical review is normally sought through the Health Research Authority for studies involving patients attending NHS clinics or users of any of the services for which UK Health Departments are responsible. This includes adult social care in England and both adult and children's social care in Wales and Northern Ireland. The Committee may consider applications involving administration of pharmaceuticals to healthy volunteers and use of NHS facilities where no patients are involved. Applications for ethical review for investigations in human biology, other than psychology, should be made to the Cambridge Human Biology Research Ethics Committee.
Guidance on policy issues may be obtained from the Committee's handbook (see 'downloads'), which contains a record of policy decisions on ethical issues, and pointers to guidance documents issued by the British Psychological Society, the Medical Research Council and the Royal College of Physicians.
In accordance with University policy, research involving human participants or personal data should not begin until proper ethical review has taken place and favourable opinion given. Retrospective ethical reviews are therefore not permitted.
It is University policy that studies involving human participants, without exception, have made arrangements for appropriate insurance. This can be obtained from the University’s Insurance Section. Applications for ethical review and insurance can be made simultaneously, but evidence of insurance (usually a letter from the Insurance Section) is needed before a letter confirming favourable opinion can be issued by the Committee. The MRC-CBU has its own insurance arrangements separate from the University, however, evidence of insurance is still needed. Studies undertaken by University staff using MRC-CBU as a site will require University insurance.
Applications for ethical review are submitted to the Committee on the application form (see box). Answer all questions using the notes provided for guidance. Append any additional information (participant information sheets, consent forms, questionnaires, adverts and so on), labelling them as appendices. Further information is given on the application form. Once complete, submissions are sent as email attachments to:
If you would like further advice on the suitability of a project for review or the review process itself, consult the ethical review process outline (on the left) or email SBS Ethics.
Applications are reviewed by two Committee members from departments other than that of the applicant(s). The Committee is constituted of senior members of departments from which applications are commonly made. The current (2024) members are:
Stephanie Archer, Department of Psychology
Tristan Bekinschtein, Department of Psychology
Isabel Clare, Department of Psychiatry
Matt Davis, MRC Cognition and Brain Sciences Unit
Michelle Ellefson, Faculty of Education
Tom Manly, (Deputy Chair), MRC Cognition and Brain Sciences Unit
Anna Moore, Department of Psychiatry
Jason Rentfrow, Department of Psychology
Helen Statham, Lay Member
Pasco Fearon, Centre for Family Research
Paul Bays, Department of Psychology
Richard Bethlehem, Department of Psychology
Tamar Makin, MRC Cognition and Brain Sciences Unit
Andres Roman-Urrestarazu, Autism Research Centre
Anna Bevan, MRC Cognition and Brain Sciences Unit
Kate Baker, MRC Cognition and Brain Sciences Unit
Sarah Lloyd-Fox, Department of Psychology
John Suckling, (Chair), Department of Psychiatry
Clinical Advisory Panel:
Kathy Beardsall, Department of Paediatrics
David Menon, Department of Anaesthesia
Ken Ong, MRC Epidemiology Unit
Matthis Zilbauer, Department of Paediatrics
Clinical Education Co-opted Members
Charlotte Tulinius, Department of Public Health and Primary Care
Anne Swift, Department of Public Health and Primary Care
Richard Darnton, Department of Public Health and Primary Care
Mark Lillicrap, ICE/Addenbrooke's
Nicola Jones, ICE/Royal Papworth
Kalman Winston, Department of Public Health and Primary Care
Jane MacDougall, Cambridge University Hospitals
Clare Morris, ICE
Appointments to the Committee are for three years. Enquiries on vacant positions should be made to the above address.